Sunlight includes UV-A, UV-B, and UV-C. A wave’s wavelength is its matching distance. UV-C (100-280nm), UV-B (280-315nm), and infrared (700-3000nm) wavelengths. Nanometers measure UV waves (nm). 1 billionth of a meter is a nanometer. Common commercial lamps produce at 365nm, 385nm, 395nm, or 405nm with UV-LEDs.UV-LED light sources have higher irradiance (W/cm2) than typical UV curing lamps due to diode and lamp advances. Commercial UV-LED lamps cure inks, coatings, and adhesives. UV-LED curing lamps emit 24W/cm2 (water-cooled) and 16W/cm2 (air-cooled) at 395nm. UV-LED 365nm systems provide 12W/cm2 (water-cooled) and 8W/cm2 peak irradiance (air-cooled).
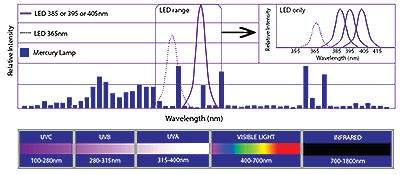
Why are we limited to high UV-A wavelength?
The properties of the diodes that are chosen during the production process of UV-LED lamp systems determine the wavelengths that are emitted by the lamp systems. There are just a few businesses, both local and foreign, that produce UV-LEDs. Applications such as vehicle illumination, general lighting, mobile devices, and signs are driving the need for LED diodes on a global scale.
In spite of the fact that UV-LED diodes have better profit margins, the market demand for them is very insignificant compared to major market drivers on a worldwide scale, such as general lighting and signs. Do UV-B and UV-C diodes exist? However, they are presently constrained by low power, an exceptionally short lifespan (less than 500 hours), and price points that may be up to 250 times more expensive than UV-A diodes.
The majority of the UV-LED development effort over the last 14 years has been concentrated on the 365 to 405 nm wavelength range because of the considerable demand for UV-A diodes in the markets that were indicated before. The performance of diodes in this area of the wavelength of UV light spectrum is well characterized, and the expected lifespan is more than 20,000 hours. It is essential to keep in mind that very lengthy lifespans are not a certainty.
Since not all UV-LED lamps are made equal, it is essential, prior to making an investment, to have a comprehensive understanding of the characteristics of the light system in question. The ongoing pricing competition in the UV-LED market is putting a damper on efforts to develop shorter wavelength UV-LED systems. Because of this, it seems likely that UV-LED products in the range of 365 to 405 nm will be the most popular ones on the market for the foreseeable future.
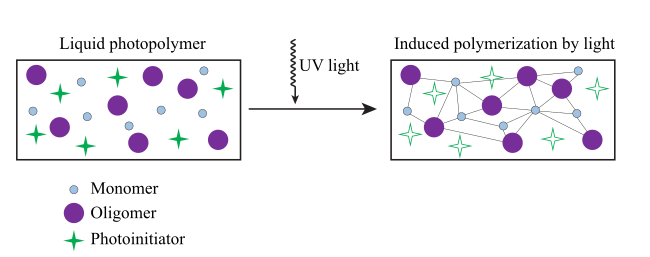
Polymerization is a process that involves the formation of extremely big molecules and molecular networks via the chemical reaction of smaller molecules to produce polymers. This process, which turns liquid formulations into a hard solid film or elastomeric solid with qualities specified for the application, is also known as curing, drying, or hardening, and is used in the coatings, ink, and adhesive industries. In the process of photo polymerization, the photons (at either UV or visible wavelengths) released by a light source are used to start chemical processes by stimulating the activity of specific additives known as photo initiators. After absorbing the UV energy, the photo initiator (PI) produces a highly reactive chemical species. This species is what starts the reactions that crosslink the resinous components (oligomers, monomers, etc.) to cure or solidify the ink, coating, or adhesive. These reactions are necessary for curing the ink and solidifying the coating or adhesive.
Related Article: Learning to use and set up Surya Professional disinfectant with UV-C sterilizer box
Formulating UV chemistries for UV-LED lamps
UV formulations for inks and coatings often include photo initiators, binders (such as oligomers or resins), diluents (such as monomers, water, or solvents), and additives. Diluents are often required to bring down the formulation’s viscosity to a level that is manageable for application by spraying, jetting, roll-coating, printing, or any number of other methods. Monomers can serve to control viscosity while also being chemically incorporated into the resulting polymer network. This results in reduced emissions and/or energy consumption in comparison to formulations that are waterborne or solvent-borne.
Monomers can take the place of water or solvents in environmentally friendly formulations. The oligomers, as well as the structure of the backbone of the oligomers, are what influence the overall quality of the material. In UV formulations, monomers and oligomers are often derivatives of acrylates or methacrylate’s, incorporating polyurethanes, polyesters, polyether’s, or acrylic chemistries. These types of acrylates and methacrylate’s are known as acrylates.
Pigments and dyes are examples of colorants that are used to add color and other effects to a formulation. Silica, waxes, clays, and extenders are used to modify the physical properties of the formulation, and sometimes they are used to lower the overall cost of the formulation by replacing some of the more costly binder components. Additives like adhesion promoters, dispersants, flow-aids, degassing agents, and UV stabilizers are used to improve certain properties of formulations while they are being physically applied and after they have been cured.
In order to produce UV curing that is both efficient and effective for an ink, coating, or glue, the formulator will attempt to match the spectrum output of the UV lamp with the absorption properties of the photo initiator (or photo initiators) that are utilized in the formulation. The quantity of PI that is typically included in a UV formulation is often rather low, falling far below 5% by weight. Broad-spectrum photo initiators (PIs) are used in the majority of present UV formulations because they are more effective at curing with standard mercury-arc lamps.
PIs usually absorb light over a wide range of wavelength of UV light rather than a single narrow band. Even though there is frequently some absorption within the UV-LED output range, it is abundantly clear that a significant portion of the conventional PI absorption range is not utilized when formulations are cured with a single-band UV-LED lamp.
This is the case even though there is sometimes some absorption within the UV-LED output range. Using a formulation that was developed particularly for UV-LED curing and a PI that had a high molar absorptivity in wavelength bands that were emitted by the UV-LED source, it is feasible to achieve a cure that is more effective. Photo initiators and other components of the formulation should be chosen so as to allow for efficient excitation of the photo initiator (or photo initiators) at wavelengths of 365 nm and 395 nm, respectively, given that the emission wavelengths of current-generation UV-LEDs are strongest at those two values.
The absorptivity (optical density) of a coating, ink, or adhesive formulation at each wavelength determines the depth of penetration into the formulation while it is curing. This may be a coating, an ink, or an adhesive. The choice of resin components, colorants, and additives that make up the formulation is what ultimately decides the optical density at each wavelength. In UV formulations, we find that UV-C wavelengths are typically absorbed in the surface layers due to the high optical density of the resins and other formulation components at the shorter UV wavelengths.
However, UV-B and UV-A wavelengths are able to penetrate more deeply into the film, even to the point where the formula meets the substrate. Therefore, the formulator is required to not only match the absorbance bands of the photo initiator to the UV emission wavelengths of the UV-LED lamp but also take into consideration the absorption characteristics of the colorants (dyes, pigments), resins (monomers, oligomers, binders), and other additives in the formulation. This is needed to avoid competitive absorption, which can stop wavelength of UV light from reaching the photo initiator.
The longer wavelength output, such as the UV-A range emissions from current high-irradiance UV-LED systems, is able to easily penetrate through thicker and more pigmented systems than the shorter wavelength UV-B or UV-C emissions. This results in through-curing of the material, which typically improves adhesion and the ability to cure thicker screen ink or pigmented wood coatings.
The shorter ultraviolet wavelengths (200 to 280 nanometers) are incapable of penetrating very far into a material; nevertheless, they can give surface curing, which is vital for qualities like scratch and chemical resistance. In some situations, the depth of penetration and degree of curing of a UV formulation can be greatly improved by changing the composition of the formulation to take advantage of the wavelength of UV light produced by the UV-LED source being used.
Related Article: Is UV-C light harmful for air ducts and electronics?
Irradiance and energy density
The degree of cure, also known as the level of reaction or crosslinking, of the components that make up a UV-cured formulation, has a substantial influence on the physical and chemical characteristics of the formulation as a whole. The degree of cure is possible for the degree of cure to vary depending on the depth of the coating or ink as well as the curing circumstances.
Nonetheless, the degree of cure is often qualitatively measured by scratch, pencil hardness, or solvent resistance. In general, the greater the number of photons that are absorbed by the photo initiators included in the formulation, the greater the number of chemical processes that take place and the greater the degree to which the substance cures, crosslinks, etc.(It is important to keep in mind, however, that more is not always better!)
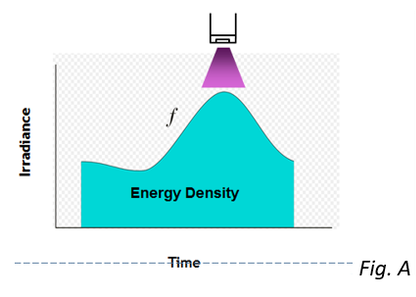
Energy density, also known as dose, is a key parameter that helps to characterize the curing conditions experienced by a UV formulation. Irradiance, also called intensity, is another important parameter that gives two specific variables that can be watched and changed to improve the overall properties and performance of a material that has been cured with ultraviolet light.
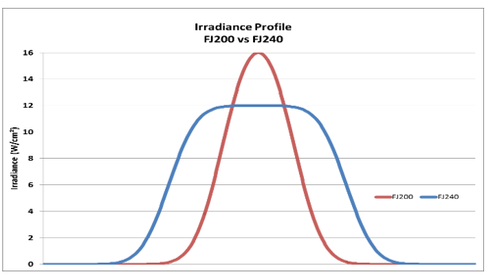
Irradiance, in its most basic form, is a measurement of the „brightness“ of the UV source as seen by the surface of the formulation while it is in the process of curing. To put it another way, energy density is determined by two factors: the „brightness“ of the UV source and the length of time the formulation is exposed to it. To be more explicit, irradiance refers to the instantaneous quantity of photons that strike the surface at a certain wavelength of UV light or range of wavelengths, and it is measured in watts per square centimeter.
The amount of irradiance that is received by the surface at its highest point during the curing process is referred to as the peak irradiance. The time-integral of irradiance is known as energy density, and it indicates the entire sum of photons of a certain wavelength or wavelength range that were received by a particular region of the surface during a particular amount of time. The Joule per square centimeter is the standard unit for expressing the energy density of a substance.
Related Article: What UV light is best for plants
Measuring irradiance
What kind of measuring instrument should be used to accurately measure the output of UV-LED bulbs? Irradiance and energy density measuring tools are available from a number of different vendors. The vast majority of today’s radiometers make use of sensors that have been described and calibrated to function with the output profiles of mercury lamps. These radiometers have not yet completely accommodated the one-of-a-kind emission characteristics of UV-LED sources.
Because UV-LEDs emit light in a highly distinctive manner, the calibration of the sensor to a certain wavelength of UV light band is the quality that is considered to be the most significant. It is possible for a radiometer to lead to measurement inaccuracies if it filters or does not count all of the UV radiation based on the typical LED wavelength tolerance. Because of this, the radiometer should not be used to determine the irradiance parameters.
The spectrum properties of UV-LED lamps are noticeably distinct from those of conventional lighting systems, and radiometers that are capable of correctly measuring UV-LED emissions have just recently become available on the market. Even so, radiometers need to be calibrated for particular properties of LEDs by certain lamp manufacturers in order to accurately measure their output. At this time, there is no such thing as a „generic“ UV-LED radiometer that can be used with many types of UV-LED lights. It is essential for original equipment manufacturers (OEMs) and end-users to employ a UV-LED radiometer that is calibrated to the standards of the UV-LED lamp source for the purpose of process control. In the absence of this, one is likely to arrive at incorrect readings and/or inappropriate conclusions.
The process of measuring irradiance is not an easy one. UV-LED lamp makers, measuring device makers, original equipment manufacturers (OEMs), and end users should all agree on a single industry standard that can be used to report irradiance and energy density data in a way that is uniform, accurate, and brief.
Related Article: Is the UV-C light leaking from my air purifier safe?
Conclusion
UV-LED lights release photons of certain wavelength of UV light, as do mercury arc lamps and microwave lamps. Photons vary solely in amount and wavelength during UV photo polymerization. UV-LED lights produce a smaller range of wavelengths than typical UV sources; therefore, formulations and radiometers must be matched to the lamp’s emission bands for maximum performance.
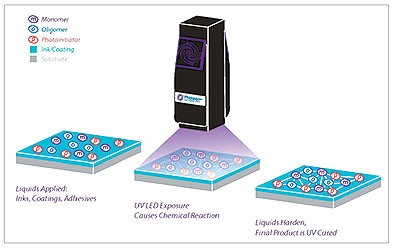
UV-LED curing is now an acknowledged, user-friendly tool in printing, coatings, and adhesives. UV-LED system features enable applications that were not feasible or constrained by traditional UV sources. Industry customers and UV-LED suppliers push formulators and chemical raw material suppliers to produce UV-LED wavelength-optimized materials and formulations. UV-LED curing devices have gotten better at sending UV light to the medium, allowing for more throughput and flexibility in the process.
Mike Higgins is Phoseon Technology’s east regional sales manager and a RadTech editor. Higgins spent nine years at Sartomer before switching to the LED sector. His background in acrylate chemistry and next-generation equipment gives him a unique view of the UV-curable business, possible uses, and future development.